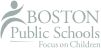Matter States and Temperature
All matter on earth exists in one of three phases or states: solid, liquid, or gas. A substance’s phase is determined by the speed of its molecular motion, often referred to as kinetic energy. Adding or removing heat energy from a substance can change it from one state to another. This course illustrates the types and properties of matter states, and concludes with a discussion of temperature scales and the different types of heat transfer.







Demos + Pricing
Learn more about our courses, get pricing, and see our platform.
Course Details
Learning Objectives
• Match each phase of matter with its characteristics • Describe the role of temperature in determining the phase of matter • Describe how heat is measured • Differentiate between the three ways that heat is transferred
Specs
Frequently Asked Questions
What are the three states of matter?
What is a solid?
What is a liquid?
What is a gas?
What is Pascal’s Principle?
What is Boyle’s Law?
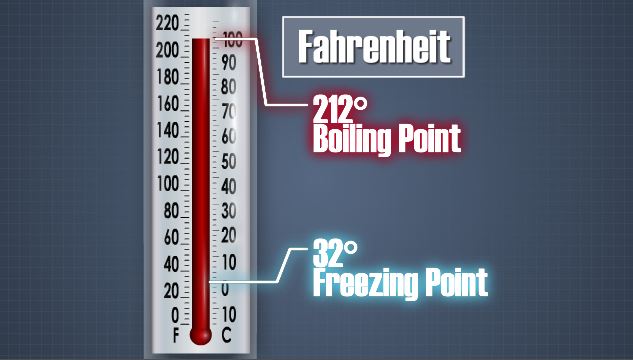
What are three temperature scales used today?
What are three ways that temperature can be transferred?
Sample Video Transcript
If the kinetic energy within a substance is so low that the attraction between the molecules is much stronger than the forces pulling the molecules apart, the material will be in a solid state of matter. The molecules have some energy but their movement is confined to vibrating or jiggling in place as they are so densely packed. Solid materials have a fixed volume and shape. They are extremely rigid and therefore cannot be easily compressed. Solids also do not flow and cannot easily be joined together with another solid. When a solid is heated to a certain point, it will change to the liquid state of matter.